BPE Plasmid Technology
Current plasmid technology manufacturing is expensive and low yielding presenting a significant challenge for applications where cost-of-goods is factor. Pegasus Biotech’s BPE plasmid technology overcomes these challenges, offering a selection-marker free, ultra-low-cost solution for our clients. The BPE uses a patented bacterial origin and genetically modified E. coli to drive industry leading yields at extremely low COGS. This platform enables rapid, plug-and-play development, allowing for the insertion of any gene of interest while maintaining excellent safety.
What is the BPE?
The BPE plasmid technology is a high yielding, ultra-low cost, selection marker free plasmid technology developed by Pegasus Biotech.
How does the BPE work?
The BPE uses a novel bacterial origin combined with a genetically modified e. coli to drive ultra-high yields in an antimicrobial free environment.
Both the origin and genetically modified bacteria are made up of IP owned by Pegasus Biotech. Replication of the plasmid is thermoregulated, allowing us to maximize fermentation yields.
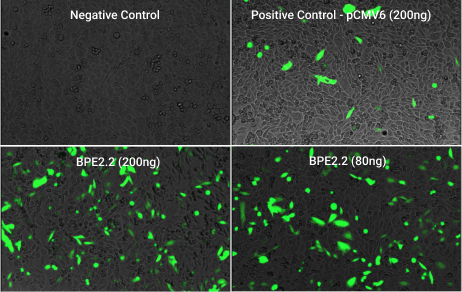
Fig 1. Increased transfection/expression of tGPF with the BPE plasmid vs. commercial plasmid.
| Characteristic | Conventional Vaccines | Conventional Plasmid Technology | Other Novel DNA Technology | mRNA | Pegasus BPE |
|---|---|---|---|---|---|
| Development Time | Long | Short-medium | Short-medium | Short | Short |
| COGS | Low | High | Very High | High | Low |
| Safety Profile | Variable | Variable | Excellent | Excellent | Excellent |
| Multivalent | Yes | Yes | Yes | No | Yes |
| Storage | Variable | 2-8°C | 2-8°C | -80°C | 2-8°C |
| Shelf Life | Variable | Long (>24 months) | Variable | Short | Long (>24 months) |
Fig 2. The BPE technology was developed to have a best-in-class combination of features for rapid development, low COGS, easy storage, and the best possible safety profile
Benefits of the BPE
| Design Requirement | The BPE Platform |
|---|---|
| Fast Development Times | Being a platform, any gene(s) of interest can be inserted into the BPE technology and produced using a plug-and-play manufacturing process and suite of pre-developed analytical methods. |
| Low COGS | The BPE was developed to be the lowest cost plasmid technology available on-market. |
| Safety Profile | By having no selection marker and minimal bacterial components the BPE technology reduces the risks posed by conventional DNA vaccine technology. |
| Multivalent | Having a backbone <1000 nucleotides, there is substantial room in the plasmid to carry multiple GOI’s. |
| Storage | Being DNA, the BPE technology is highly stable at 2-8ºC. |
| High Expression | The BPE technology was developed to have industry leading expression of the GOI. |
Quantification of BPE Expression
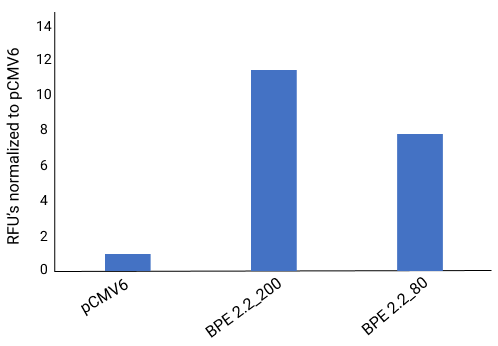
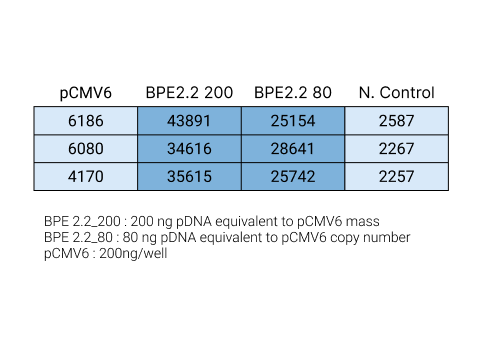
Performance
Cells were transfected with a BPE plasmid carrying a tGFP gene at the same number of copies/cell and at the same mass of plasmid/cell. The BPE plasmid had 4X the expression at the same numbers of copies/cell, and 7X the expression when transfected at the same mass of plasmid/cell. In real world applications, by being able to use a lower dose can drive extremely low COGS.
